- Trutect™, formerly known as Elanco’s Canine Parvovirus Monoclonal Antibody (CPMA), was conditionally approved by U.S. Department of Agriculture (USDA) for use in May 2023 and has since helped save thousands of puppies across the country.
- 93% of puppies treated with CPMA survived in real-world usage[i] and when treated with CPMA, parvo patients spend on average 1.87 less days in the hospital.[ii]
- In June 2025, the USDA approved the product for passive immunity, allowing veterinarians to protect exposed puppies from canine parvovirus as a valuable, preventative option.
- Elanco is proud to offer a $200 rebate on treatment for pet owners whose puppies have been infected or exposed to parvo.
INDIANAPOLIS, Ind. (Dec. 15, 2025) – Elanco Animal Health today announced that its lifesaving treatment for the deadly canine parvovirus (parvo), Canine Parvovirus Monoclonal Antibody (CPMA), has received full approval from the U.S. Department of Agriculture (USDA) and will join Elanco’s Tru portfolio under the new brand name Trutect™. Parvo is a devastating disease that affects more than 330,000 puppies annually, with up to a staggering 91% mortality rate without supportive care.[iii],[iv] Prior to Elanco’s monoclonal antibody, the only treatment for parvo was supportive care. In 2023, the USDA provided a conditional license for CPMA as the first and only approved therapeutic solution to treat parvo.
“Receiving full USDA approval of our lifesaving parvo treatment reinforces our promise to go beyond for veterinarians, pet owners, and, most importantly, the puppies across the country who need it most,” Bobby Modi, Executive Vice President, U.S. Pet Health and Global Digital Transformation at Elanco. “Under the brand name Trutect, our mission remains the same, to save puppies from parvovirus.”
From the Start, CPMA (Now Trutect) Has Helped Defend Puppies. Defeat Parvo.
Since launching Trutect in May 2023 under the CPMA name, Elanco has helped create broader awareness of this devasting disease and saved thousands of puppies from parvo thanks to this first-of-its-kind product. In 2023, Cookie, an eight-week-old pit mix in California became one of the first dogs to receive the innovative treatment.
In 2024, Elanco launched a multi-year mission aimed at helping defend puppies and defeat parvo and launched the first-ever National Parvo Awareness Day aimed at raising awareness and stopping the spread of parvo. Additionally, Elanco has completed expansion of and will continue investing in the Elwood, Kansas manufacturing facility to further grow the company’s monoclonal antibody (mAb) platform.
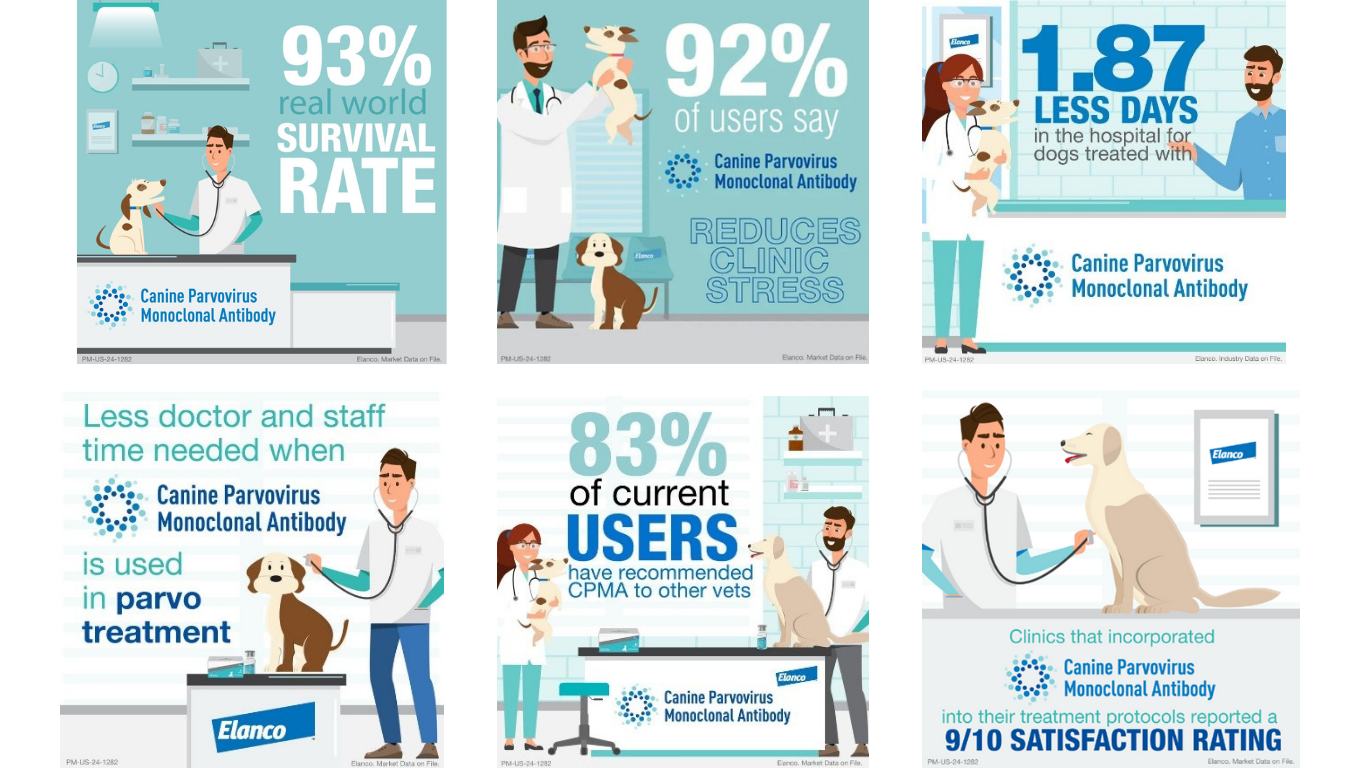
In the first year of launch, real-world data showed that:
- 93% of puppies treated with CPMA survived in real-world usage.i
- When treated with CPMA, parvo patients spend on average 1.87 less days in the hospital.ii
- 92% of veterinarians using CPMA report that the treatment reduces overall clinic stress.i
- A 90% satisfaction rating among clinics who incorporated Canine Parvovirus Monoclonal Antibody into their treatment protocols.i
- 83% of those who have used the Canine Parvovirus Monoclonal Antibody recommend it to their peers.i
“The real-world data collected over the first year of CPMA availability, shows that as a result of these efforts and more, survival rates for puppies battling the deadly virus improved. We also saw enhanced operational efficiency and high satisfaction within veterinary clinicsi – demonstrating that Trutect has already started re-writing the protocol for parvovirus treatment,” said Dr. Jill Pattee, veterinarian at Elanco Animal Health.
Expanding Access to Trutect
Earlier this year, Elanco donated more than $3 million of the product to 2,300 clinics and shelters in high parvo areas across the country. In addition to its commitment to expanding the availability of the product across the country, Elanco is also proud to offer a $200 rebate for pet owners whose puppies have been infected or exposed to parvo.
In June, the USDA approved the use of CPMA (now Trutect) for passive immunity (i.e., prophylactic treatment) to prevent parvo infection in puppies exposed to the virus, expanding the potential to protect dogs from this devastating disease. A recent clinical study that found that when given at a prophylactic dose, CPMA was 100% effective in providing passive immunity to healthy dogs 8 weeks of age or older against parvo.[i]
“The recent clinical study on CPMA’s ability to prevent parvo infection when administered prophylactically resulted in no CPMA-treated dogs developing parvo infection, highlighting its power to provide passive immunity and effectively stop parvo before it starts,” said Dr. Jill Pattee, veterinarian at Elanco Animal Health. “This evidence-based approach offers a crucial tool for safeguarding vulnerable young puppies against this highly contagious and often devastating disease.”v

“As an emergency room veterinarian, I have seen firsthand the devastating impact of parvo throughout my career,” said Dr. Tannetje Crocker, emergency veterinarian at Veterinary Emergency Group in Dallas, TX. “Since 2023, I have proudly used CPMA and have reunited countless puppies with their families. I’ve also used the product on exposed puppies, helping to keep them protected from this highly contagious virus. I believe every vet clinic should keep Trutect on hand because you never know when a parvo puppy will need this targeted treatment.”
To learn more about Trutect visit DefeatParvo.com.
ABOUT ELANCO
Elanco Animal Health Incorporated is a global leader in animal health dedicated to innovating and delivering products and services to prevent and treat disease in farm animals and pets, creating value for farmers, pet owners, veterinarians, stakeholders and society as a whole. With 70 years of animal health heritage, we are committed to breaking boundaries and going beyond to help our customers improve the health of animals in their care, while also making a meaningful impact on our local and global communities. At Elanco, we are driven by our vision of Food and Companionship Enriching Life and our purpose – all to Go Beyond for Animals, Customers, Society and Our People. Learn more at www.elanco.com.
PM-US-25-1724
[i] Elanco Animal Health. Market Data on File.
[ii] Elanco Animal Health. Industry Data on File.
[iii] Elanco Animal Health. Data on File.
[iv] Sykes, JE. Canine Parvovirus Infections and Other Viral Enteritides. Canine and Feline Infectious Diseases. 2014:141-151
[v] Elanco Animal Health. Data on File.
